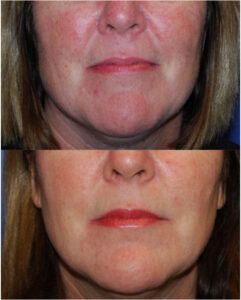
The term “off-label treatment” may conjure ideas of something experimental, secretive, or even potentially dangerous. In reality, however, off-label use of relatively new products or technologies is often remarkably effective and inventive in the hands of an experienced, responsible clinician. So let’s explore the topic of off-label treatments.
When a drug or treatment is first developed by medical companies, a clear and singular application is targeted. “Botox cosmetic® is indicated for treatment of moderate wrinkles between the eyes (glabella) for patients aged 18-65, for a duration of 3-6 months.” All research specific to this use is then included in their approval process to the FDA. Once approved, this becomes the only situation officially sanctioned as safe and effective by the FDA, and therefore is the on-label indication. This process costs the medical companies millions of dollars in research, development, administrative and legal fees. It also severely limits initial patterns of use despite broader applications of drug or treatment options.
The term off-label use then applies to any other site, age group, location, or other application of the drug or treatment. For our Botox cosmetic® example above, that means using the medication to treat crow’s feet, bunny lines, smoker’s lines, or forehead creases. We know that Botox cosmetic® is remarkable successful and effective for such use, even though it was initially “off-label.” Soft tissue fillers were initially indicated for the nasolabial folds, but they are used “off-label” daily for lip, marionette lines, cheeks, scars, wrinkles, and jowls.
The point is that off-label use is first a by-product of the narrow FDA approval process. Second, a thorough understanding of the products and their mechanism of action allow the thoughtful and inventive physician to apply on-label success to expanded situations. Third, data is then collected from off-label use and used to repetition the FDA for expanded indications. That’s why Botox cosmetic® is now FDA approved and indicated for crow’s feet and migraine treatments, and why Restylane® is additionally approved for lip enhancement. And finally, the millions of dollars spent on marketing these great products all highlight on-label situations.
So don’t get tripped up or freaked out by the term “off-label.” When you trust your appearance to the experts at RENEW and RPS, you have our guarantee of the highest standards of patient care and treatment results. And our involvement with off-label use will always be supported by years of experience balancing cutting edge technology with scientifically proven results.